Pyridine and its derivatives exhibit ubiquitous applications across pharmaceuticals, agrochemicals, and biologically active molecules. Within the realm of pyridine functionalization research, ortho- and para-positional modifications are extensively explored and well-documented; however, the targeted meta-positional functionalization remains an elusive and formidable challenge for the scientific community. Traditional methods often rely on transition-metal catalysis, requiring harsh reaction conditions and limited substrate scopes, thus constraining their utilization in drug development.
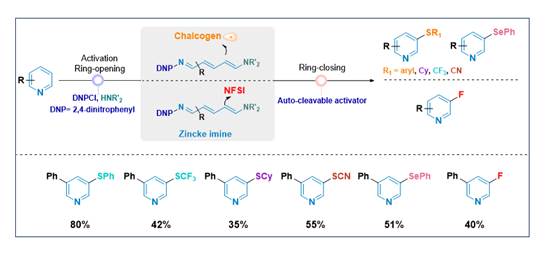
Recently, Haiyan Fu and Hua Chen et al. in the College of Chemistry, Sichuan University has made a great achievement in this field by utilizing a sequential process of pyridine ring-opening, functionalization, and ring-closure via the N-2,4-dinitrophenyl (DNP) Zincke imine intermediate. For the first time, they have selectively accomplished arylthiolation, trifluoromethylthiolation, alkylthiolation, thiocyanation, selenation, and fluorination of the C3-H bond of pyridine under mild conditions with high selectivity. Through radical inhibition and trapping experiments, coupled with DFT theoretical calculations, they have confirmed that the former reactions (arylthiolation, trifluoromethylthiolation, alkylthiolation, thiocyanation, and selenation) proceed via a radical addition-elimination pathway, whereas fluorination follows a two-electron electrophilic substitution route. This strategy significantly broadens C3-H functionalization in pyridine, providing an efficient route to diverse derivatives crucial for drug chemistry and functional materials research.
This work has been published in Nature Communications with the title "C3 Selective Chalcogenation and Fluorination of Pyridine using Classic Zincke Imine Intermediates,". Sichuan University is top institute in the address list. Prof. Haiyan Fu and Hua Chen are corresponding authors, and the doctoral student, Shun Li is the first author. The DFT calculations were carried out by Professor Zhishan Su's team. This research was financially supported by the National Natural Science Foundation of China and the Science and Technology Department of Sichuan Province. The authors also express gratitude to Chunchun Zhang from the Centre of Analysis & Testing, Dongyan Deng, Jing Li, and Meng Yang from the College of Chemistry, Sichuan University, for conducting NMR, HRMS measurements, and X-ray structure determination (Article link: https://doi.org/10.1038/s41467-024-51452-0).
